$2.22 +0.0200 ( +0.92% ) 20.8K
NASDAQ: GENE
Company Overview
Genetic Technologies is a diversified molecular diagnostics company. A global leader in genomics-based tests in health, wellness and serious disease through its geneType and EasyDNA brands. GENE offers cancer predictive testing and assessment tools to help physicians to improve health outcomes for people around the world. The Company has a proprietary risk stratification platform that has been developed over the past decade and integrates clinical and genetic risk to deliver actionable outcomes to physicians and individuals. Leading the world in risk prediction in oncology, cardiovascular and metabolic diseases, Genetic Technologies continues to develop risk assessment products.
Core Focus
- Execute B2B commercialization of geneType multi-risk test
- Demonstrate clinical validity & clinical utility of geneType tests
- Drive EasyDNA & Affinity DNA revenue growth: tests, channels & markets
- Innovation: developing next generation of capability, starting with epigentics
Value Proposition
Genetic Technologies is executing a B2B commercialization strategy for its flagship geneType multi-risk test covering breast cancer, colorectal cancer, prostate cancer, ovarian cancer, coronary artery disease and Type-2 diabetes, a first-in-class test that can predict a person’s risk in up to 70% of annual mortalities and morbidities before onset. The Multi-Risk test, along with integration of recently acquired DNA based products, underpin a broad and complementary portfolio of genomic based tests creating a significant competitive advantage. GENE’s expanding product portfolio includes more than 50 risk assessment tests in 14 test categories covered by 25 patents granted and nine patents pending. In addition to its B2B model, GENE is also focused on expanding its direct-to-consumer testing programs. The global market for predictive genomics is expected to reach $4.6 billion by 2025, growing at a CAGR of 17%+.
Collaborations
- Memorial Sloan Kettering Cancer Center
- Harvard Medical School: Prof. Bernard Rosner
- Washington University St Louis: Prof. Graham Colditz
- University of Melbourne: Prof. John Hopper & Prof. Jon Emery
- Ohio State University
Market Data
Investment Highlights
- Executing our Vision and Strategy to be a leader in personalized predictive genomics
- Our journey from R&D → Commercialization and the pathway to profitability
- Milestones include:
- Launched our NEW digital transformation strategy
- We’ve never seen an explosion of interest like this in the history of the company
- “Know your Risk” women's health event in the U.S. Co-Hosted by Krystal Barter and Dr Kristi Funk
- Launching the development of world’s most comprehensive risk test
- Major breakthrough - geneType predicting risk of Pancreatic Cancer
- Industry Partner in Multi-Cancer Risk trial
- New Market opportunities in Europe, S.E.A. and UK
- Engaged with leading global collaborations
- Continuing our journey with a strong commitment to ESG principals
- Have a well-defined strategic plan to execute on a multi- brand strategy in key regions
Annual Results - Momentum Building on our Commercial Plans
Genetic Technologies Limited has had an exciting period of commercial growth over the last 12 months in our Business to Business (B2B) channel led by geneType and our Direct to Consumer (DTC) channel led by our EasyDNA and Affinity DNA brands. Our commitment to transforming healthcare through personalized genetic insights continues driving meaningful advancements, revolutionizing medical practices across the globe.
This year we have continued the transformational journey from an R&D organization with one polygenic risk test to an organization with revenues anchored in 3 brands: geneType, EasyDNA, and AffinityDNA.
Highlighting our commercial progress, the company announces revenues for the 12 months ending June 30, 2023, of A$8.686million, an increase of +A$1.891m; +28% compared with 2022.
Momentum building - Growth and Partnerships:
The dedication of our team, and the power of geneType, have ignited a revolution in healthcare. We are seeing encouraging growth in our commercial volumes for geneType, a testament to the value and trust we are building in the medical community. Twenty medical practices in both the US and Australia are now routinely referring patients for geneType testing, with new practices joining our network every week. This is laying the foundation for a healthcare landscape that embraces precision medicine and individualized care.
Empowering Precision Medicine Centers:
Our vision extends beyond testing; we are leading the way establishing partnerships in Precision Medicine Centers of Excellence. Paving the way for a transformative approach to patient care, harnessing the potential of geneType, creating centers that redefine medical practices and deliver more precise diagnoses and personalized treatment strategies.
Innovating for the Future:
We were proud to announce the development of the world’s first Comprehensive Breast & Ovarian Cancer Risk Test, a true testament to our dedication to innovation. This groundbreaking test, showcased at the annual BRCA (BReast CAncer) conference in Montreal, will empower women to understand their risk profile for these deadly diseases through a simple saliva sample.
Pioneering Research and Clinical Impact:
Our ground-breaking work is gaining recognition on a global scale. The science team have successfully published seven peer reviewed manuscripts and publications in prestigious journals across the world. These publications provide the evidence that the implementation of the patented geneType test will identify more at-risk patients, detect the disease early and save lives.
Expanding the Frontiers of screening:
Our geneType Multi-Test Panel now encompassing an impressive nine life threatening diseases. In phase 2 of our rollout plan, we have expanded the panel to include melanoma, pancreatic cancer, and atrial fibrillation. This expansion enhances the test’s utility, offering risk assessments for six cancers, two cardiovascular diseases, and one metabolic disease, all from a single saliva sample, covering up to 70% of mortalities and morbidities annually.
Championing Partnerships:
Our strategic alliance with QIAGEN marks a monumental step forward. Together, we are establishing a “Centre of Excellence” facility in Australasia, uniting our life science and diagnostics expertise. This partnership amplifies our capabilities, driving commercial opportunities, enhanced automation, and increased capacity. Our shared goal is to make geneType accessible to a wider audience, accelerating the identification and intervention for at-risk patients.
Our Medical Leadership:
Dr. Joel Evans joined our team in the U.S. and is a leader in clinical and functional medicine whose expertise and passion for personalized medicine align perfectly with our mission. His appointment underscores our commitment to bringing cutting-edge medical insights to our patients, reinforcing our dedication to advancing healthcare.
None of our achievements would have been possible without the unwavering support of Key Opinion Leaders (KOLs) who are driving change in their fields. Their endorsement of geneType has opened doors to new medical practices, expanding our reach and impact. We are honored to collaborate with KOLs such as Dr. Carolyn Young, Dr. William Stanford, and Dr. Lisa Larkin, whose contributions are shaping the future of healthcare.
Looking Ahead:
As we reflect on these remarkable milestones, we are invigorated by the promise that lies ahead. The journey of GeneType Group is one of transformation, empowerment, and relentless pursuit of a healthier future. We are pioneering a new era of healthcare, where every patient’s genetic makeup is harnessed to inform personalized treatments and interventions. Thank you for being a part of our incredible journey.
Our team has dedicated two decades to relentlessly advancing the forefront of genetic testing, introducing the most innovative solutions to the global stage. Over the last 18 months, Genetic Technologies has transitioned to an organization earning significant revenues and have identified the pivotal clinical and commercialization pathways for continued success. Looking ahead to the next 18 months, we are embarking on a determined journey towards attaining profitability.
- FY23 revenue up 28% YoY to $8.7 million (AUD)
- geneType Multi-Risk test now implemented in 64 clinics
- Peer-reviewed publication confirms geneType risk test outperforms traditional risk assessments for breast cancer by up to 9x
- Targeting 10k general practitioners across Australia
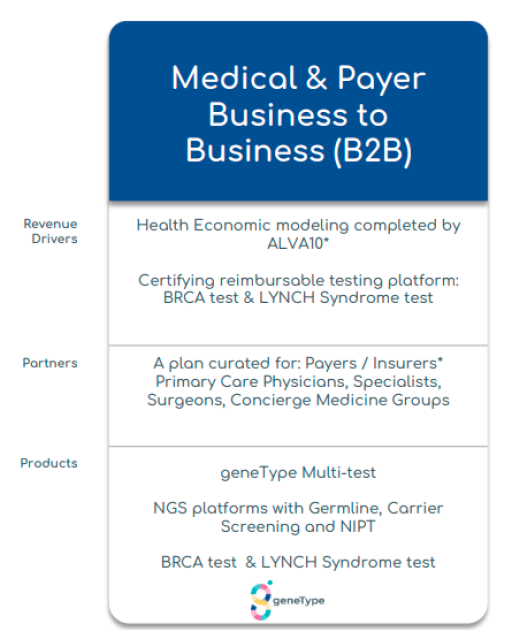
- Material progress for commercial rollout in US with Alva10 & large payer engagement
- New US business manager gaining traction with concierge medicine groups & independent doctor networks
Comprehensive Genomics-Based Testing via Multi-Brand Strategy

geneType Pathway to Market
Articles
Genetic Technologies (NASDAQ: GENE): A Visionary in Precision Medicine and Cancer Risk Assessment
Jan. 30, 2024
Genetic Technologies Limited (NASDAQ: GENE) emerges as a visionary in the realm of precision medicine and cancer risk assessment, showcasing a commitment to innovation and groundbreaking achievements. The company's recent developments underscore its pioneering role in genomics-based testing for health, wellness, and serious diseases, making it an enticing prospect for investors.
Read MoreGenetic Technologies: Innovating Precision Medicine and Revolutionizing Disease Detection
Jan. 30, 2024
Genetic Technologies Limited (NASDAQ: GENE), at the forefront in personalised predictive genomic, is reshaping the healthcare landscape with its inventive strategy for early disease detection and personalized wellness. Positioned as a diversified company, GENE's pioneering initiatives in genomics-based tests place it at the forefront of precision medicine, presenting substantial opportunities for investors.
Read More